EU4MEDTECH – revolutionizing the evaluation and regulation of innovative medical devices and in vitro diagnostic tools across Europe!
U4MEDTECH is an ambitious Horizon Europe initiative aimed at revolutionizing the evaluation and regulation of innovative medical devices and in vitro diagnostic tools across Europe. The project introduces the EU4MEDTECH Framework, a comprehensive and lifecycle-oriented approach that supports the generation, evaluation, and validation of clinical and performance evidence for high-risk medical devices and software.
With a consortium of 14 international partners and a project duration of 48 months, EU4MEDTECH is committed to strengthening the EU’s regulatory framework to ensure patient safety and facilitate innovation in the health technology sector.
Each partner contributes to the research, development, validation, and implementation of the EU4MEDTECH framework, fostering a more efficient, standardized, and innovation-driven environment for medical technologies in Europe.

Central to the project is the development of an interactive, cloud-based digital platform that integrates advanced tools for regulatory communication, global regulatory search, user training, and data repositories. This platform will be tested and validated through three practical use cases, each targeting critical segments of medical technology.
By fostering collaboration among key stakeholders, from researchers and regulators to healthcare providers and policymakers, EU4MEDTECH aims to create a sustainable and scalable ecosystem for medical device evaluation in Europe and beyond.
![]()
Main Results
EU4MEDTECH Framework
A lifecycle-oriented framework for generating and evaluating clinical and performance evidence of high-risk medical devices. It includes standardized methodologies, multi-domain criteria, and tools for both pre- and post-market stages.
Interactive Digital Platform
A cloud-based platform operationalizing the EU4MEDTECH Framework. It features a regulatory communication channel, global regulatory search functionality, a user training module, and a data repository to streamline medical device evaluation processes.
Validated Use Cases
Application and testing of the framework in three distinct use cases: Class III and Implantable Devices. Class C/D In Vitro Diagnostics. Software-based Medical Devices and Innovative Tools.
Comprehensive Exploitation Roadmap
A strategy ensuring widespread adoption, scalability, and sustainability of project results, supported by stakeholder engagement and co-design principles.
Clinical Studies and Proof-of-Concept Evaluation
Conducting three clinical studies and a proof-of-concept evaluation of the digital platform to validate its effectiveness in real-world scenarios.
Recommendations for Common Specifications
Development of new guidelines and specifications to enhance the regulatory environment, fostering innovation while maintaining safety and compliance.

EU4MEDTECH Establishes the Stakeholders Forum to Guide Strategic Project Development
The EU4MEDTECH project has reached a major milestone with the establishment of its Stakeholders Forum – a multidisciplinary collaborative platform that brings together organizations and experts that will play a central role in shaping the project’s direction and outcomes.
By involving a broad spectrum of stakeholders, the EU4MEDTECH Stakeholders Forum ensures that diverse, real-world perspectives are embedded throughout all phases of project development. The Forum is structured around two main categories – direct and supporting stakeholders.
Direct stakeholders are actively involved in the innovation life cycle of medical devices (MDs) and in vitro diagnostics (IVDs), and include five core groups:
- Researchers & Developers, who drive innovation and conduct studies to advance MedTech solutions;
- Medical Device Companies (MDCs), responsible for the design, manufacturing, commercialization, and advocacy for MDs/IVDs;
- Notified Bodies & National Competent Authorities (NBs & NCAs), who assess, certify, and ensure regulatory compliance;
- Health Technology Assessment (HTA) Bodies, that evaluate the value and cost-effectiveness of technologies for policy and reimbursement decisions; and
- Healthcare Providers & Patients/Citizens, who use the technologies in practice and ensure patient needs and perspectives are considered.
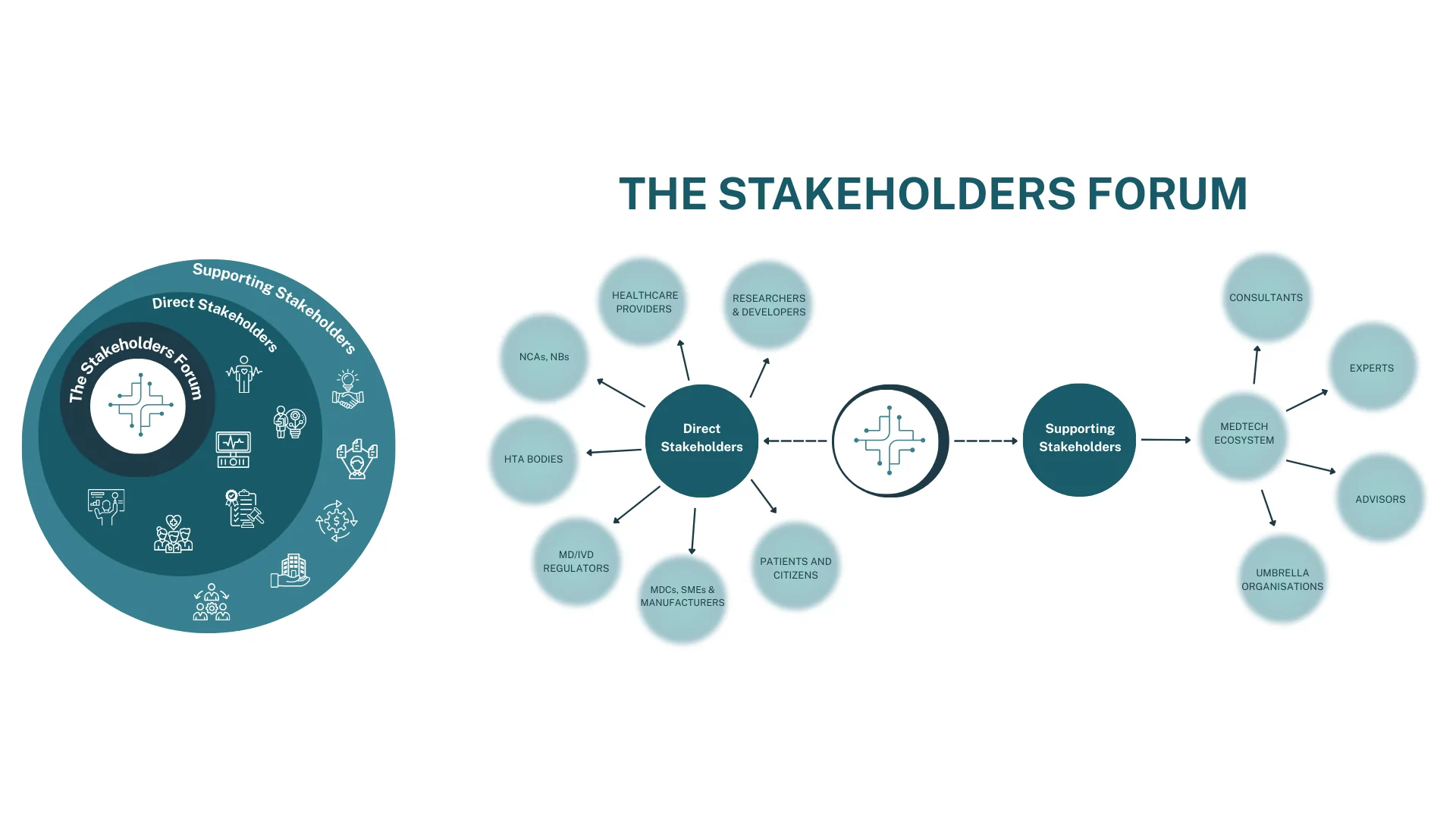
Supporting stakeholders, while not directly engaged in the innovation cycle, bring critical expertise and advisory input. These include experts, consultants and umbrella organisations who contribute with their domain knowledge and broad oversight across the MedTech ecosystem.
As a structured platform bringing together experts, the Forum will provide targeted feedback, recommendations, and validation at key project milestones. This collaborative mechanism will be instrumental in refining the EU4MEDTECH framework, enhancing the digital platform through iterative feedback cycles and steering the project toward outcomes that are both evidence-based and feasible for practical application.
Get involved with EU4MEDTECH!
The EU4MEDTECH Stakeholders Forum is a growing community. Whether you are part of one of the stakeholder groups or simply passionate about improving the regulatory landscape for high-risk and innovative medical devices and in-vitro diagnostics – your involvement is welcome!
You can join the Stakeholders Forum and subscribe to the EU4MEDTECH newsletter to stay informed about upcoming engagement opportunities by clicking the link.
https://eu4medtech.eu/stakeholders-forum/

MedTech Europe joins the EU4MEDTECH Stakeholders Forum
We are proud to welcome MedTech Europe as a member of the EU4MEDTECH Stakeholders Forum!
As the leading European trade association representing medical technology industries – from diagnostics, medical devices and digital health – MedTech Europe plays a central role in shaping health innovation policy across Europe. With its broad membership base and deep expertise in policy and regulatory affairs, digital health, and market access, MedTech Europe is an essential partner in discussions about the future of medical device (MD) and in-vitro diagnostics (IVD) regulation.
“We’re pleased to support EU4MEDTECH’s efforts to provide practical tools to strengthen the regulatory science behind clinical and performance evaluation of medical devices and in vitro diagnostic medical devices,” said MedTech Europe Director of Regulatory Affairs Petra Zoellner. “A future-fit legal framework must be efficient, predictable, and innovation-friendly. One that ensures patient safety while enabling timely access to technologies across Europe. We look forward to contributing our expertise to help co-create solutions that work for patients, health systems, and innovators alike.”
Their involvement in the Stakeholders Forum marks a significant milestone and strengthens EU4MEDTECH’s ambition to develop smarter, evidence-based, and innovation-supportive regulatory approaches. Engaging key players like MedTech Europe helps ensure that the project outputs are relevant, feasible, and aligned with the realities of the MedTech sector.